The Problem
Many times do we hear of the sad story of loss due to delayed or mis-diagnosis. Mis-diagnosis is one of leading causes of preventable deaths in sub-Saharan Africa. A big contributing factor to this is the lack of quality diagnostics infrastructure and human capital.
Some Data
• < 10% of sSA labs outside South Africa are accredited to internationally recognized quality standards
• Average of 1.7 quality accredited labs per million people in sSA
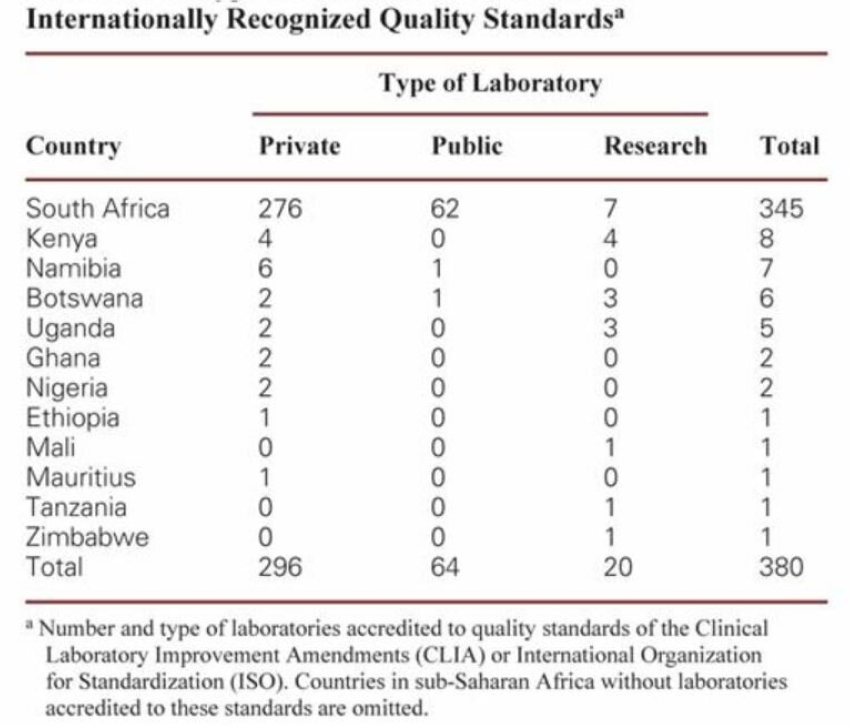
Reference:
Lee F Schroeder MD/PhD, Amukele Timothy MD/PhD. Medical Laboratories in sub-Saharan African that meet international quality standards. American Journal of Clinical Pathology, Volume 141, Issue 6, June 2014, Pages 791–795
Our Approach – Creating an effective high quality lab ecosystem

1. Map + Plan
We conduct assessment and mapping of laboratory infrastructure relative to population in low-resource regions to determine optimal diagnostics volume re-distribution.

2. Build/Acquire + Certify
We acquire and re-design or build low-footprint modular laboratories, forward stocking locations, and cold-chain facilities for remote and low volume clinical sites. We subsequently enroll in CAP proficiency program towards accreditation.

3. Hub/Bank + Distribute
We hub specialized testing in reference labs. Our labs have lower cost benefits due our Group/bulk purchasing power. We also provide Biospecimen procurement services for consented and/or IRB/ethical committee approved Biospecimen banking protocol

4. Recruit + Train
We recruit and train our associates to international standards. We sponsor associates to attain local and international laboratory and project management professional certifications

5. Test + Data mine
We use best-in-class laboratory technology and assays to deliver quality and reliable results to our affiliate clinical sites. We provide data and analytics report to affiliates to enable public health surveillance and support precision medicine.

6. Research + Develop
We conduct clinical programs and contract research for global biopharma clients who are looking to develop inclusive precision medicine or CDx. We perform cost-effective validation studies for clients seeking FDA 510(k) clearance.