Authors: Oluwatosin Idowu, Anthony Olayemi, Nzube Ekpunobi
Cancer remains one of the world’s most pressing health challenges, with nearly 10 million deaths reported in 2022 and cases projected to rise dramatically in the coming decades [1]. Early detection is universally recognized as the most effective way to reduce this burden. Yet, traditional methods of screening and diagnosis often detect cancer only after symptoms appear, when treatment options are limited and outcomes are poorer. This is where biomarkers represent a transformational shift.
As measurable indicators found in blood, tissue, or other bodily fluids, biomarkers could give a window into the earliest biological changes that precede cancer symptoms. Their potential to move oncology from reactive to proactive medicine positions them as one of the most powerful tools we have to save lives and cut healthcare costs. But the promise of biomarkers is not yet evenly realized.
Despite remarkable advances, there are significant gaps between potential and practice. Underrepresentation of genetically diverse populations, especially within Africa, threatens to limit discoveries that could benefit the entire world. Access to advanced diagnostic technologies such as liquid biopsies and genomic profiling remains concentrated in wealthier countries, leaving many patients excluded from the very breakthroughs that could transform their care [3].
At the same time, the high cost of biomarker testing, alongside reimbursement challenges and regulatory hurdles, poses barriers even in developed healthcare systems [5].
What then is the hope of harnessing the potential of these advancements in resource-poor settings? If these scientific breakthroughs are still in their infancy, perhaps it is the perfect opportunity to correct the mistakes of the past regarding diverse representation and do it the right way from the start.
This article explores how biomarkers are reshaping cancer detection, why inclusivity in research is a scientific and moral imperative, and how innovations in diagnostics and tech can close the gap between what is possible and what is practiced.
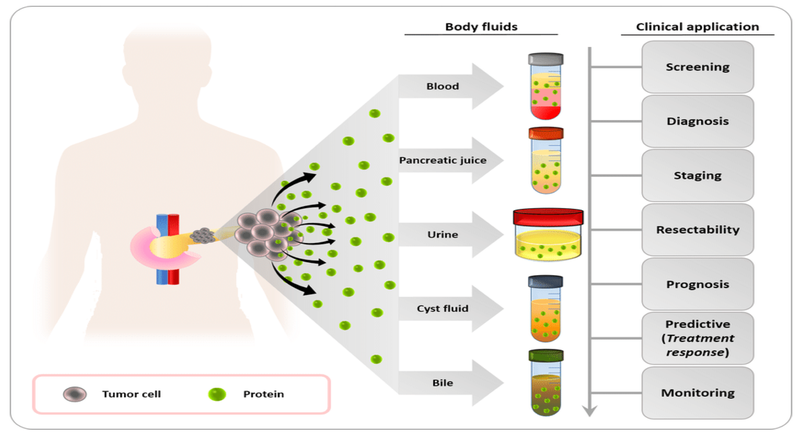
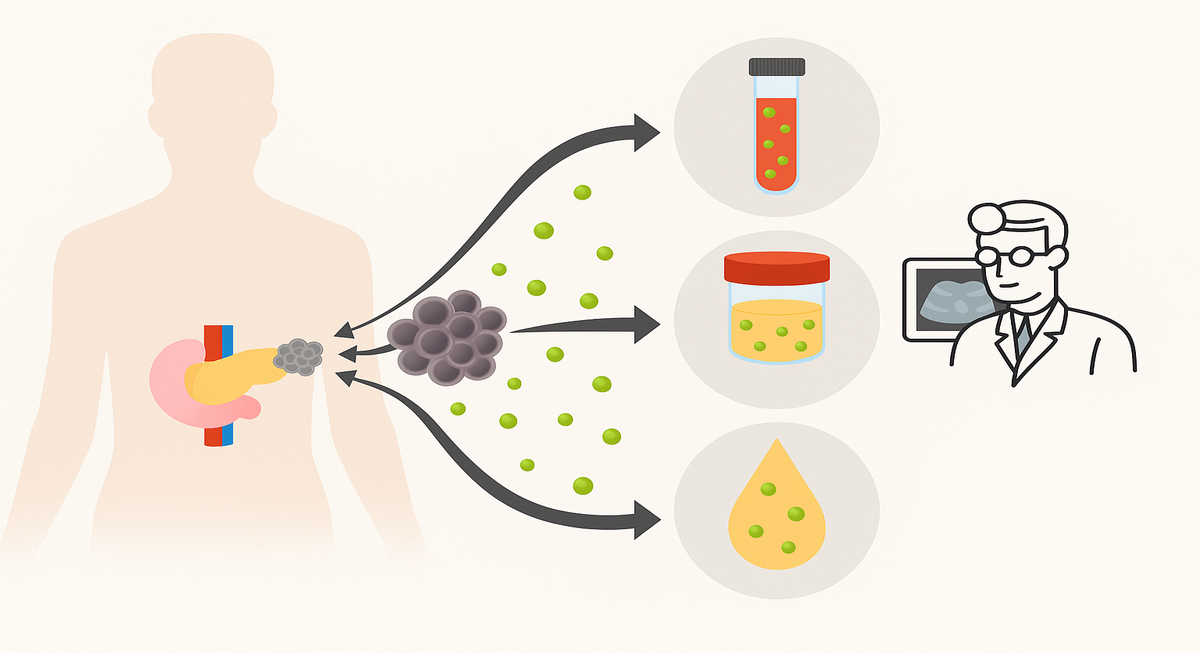
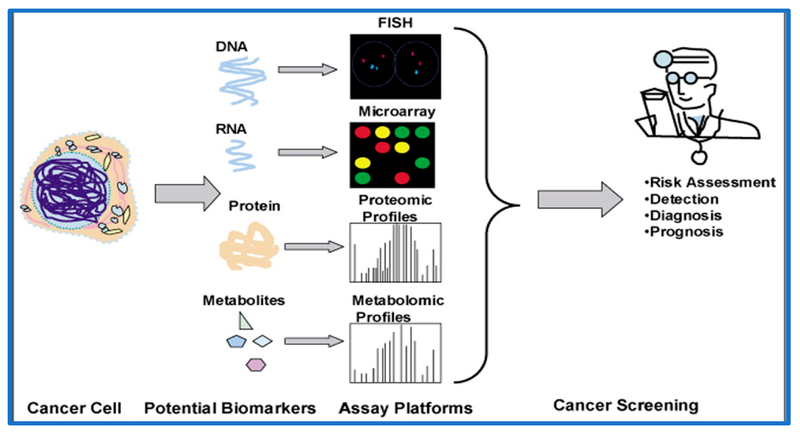
Biomarkers provide valuable information about a disease. In cancer, these may be proteins, genetic mutations, or other molecules that signal the presence of malignant cells in the body. They can be found in blood, urine, saliva, or tissue samples, and their presence often correlates with tumour growth, metastasis, or the body’s immune response (Figure 2) [6].
Well-known examples include Carcinoembryonic Antigen (CEA) in colorectal and pancreatic cancer, CA-125 in ovarian cancer, Alpha-fetoprotein (AFP) in liver and testicular cancers, Prostate-Specific Antigen (PSA) in prostate cancer, and circulating tumour DNA (ctDNA), which is being investigated as a non-invasive marker for mutations and minimal residual disease.
Still, even the most established biomarkers have limitations. For instance, CA-125 is widely used in ovarian cancer detection but suffers from poor specificity [7].
In oncology, “early detection” means identifying cancers at their earliest, pre-symptomatic stages (Stage 0 or I), when treatment outcomes are far more favorable. This emphasis is critical because many biomarkers currently in use are more reliable for diagnosis after symptoms, or for prognosis and monitoring response to therapy, rather than for pre-symptomatic screening.
As highlighted in a 2023 review, most blood-based assays perform best in late-stage cancers, not in early disease [8]. Uttley et al. similarly noted in 2016 that, despite hundreds of candidate biomarkers being described, only a handful have undergone sufficient validation for population-level screening [9]. Liquid biopsy approaches using ctDNA, while promising, remain costly and largely inaccessible for routine screening, even in high-income countries.
Globally, researchers are pursuing strategies to overcome these challenges by balancing sensitivity and specificity. One model proposed by Cree in 2011 pairs a highly sensitive but low-specificity test for broad screening with a second, highly specific assay for confirmation [10]. Uttley et al. emphasize that this two-step strategy could make early detection both feasible and cost-effective [9].
Ovarian cancer illustrates this principle. By combining CA-125 with HE4 (human epididymis secretory protein 4) or menopausal status, multimarker panels improve sensitivity without sacrificing specificity [7]. Similar innovations are underway in other cancers. The use of liquid biopsy panels in bladder cancer and metabolomic profiling in lung cancer suggests a future where early detection is not dependent on a single marker but on integrated biomarker strategies [8].
In Nigeria and across much of Africa, the translation of biomarker advances into practice is uneven. Some markers, like AFP and PSA, are more commonly requested in clinical settings than newer molecular assays. However, availability remains constrained even at the highest levels of care.
A 2022 survey of 34 government-owned tertiary hospitals found that while nearly 80% could perform total PSA tests, fewer than half had facilities for free PSA testing, which is necessary for improving accuracy and avoiding unnecessary biopsies [12].
If such gaps exist in tertiary care, it is unlikely that lower-level general hospitals or community clinics, where most patients seek care, can offer reliable access. This reflects a broader systemic barrier because while global biomarker science advances rapidly, infrastructure, costs, and training limitations in Nigeria restrict its translation into accessible early detection tools.
Despite the widespread use of biomarkers in precision oncology, one of the major challenges remains the underrepresentation of genetically diverse populations in research [13]. Africa remains the most genetically diverse continent, and biomarkers validated in Europe, North America, or Asia may not perform the same way in African populations.
The implication is that adopting biomarker-based strategies in Africa cannot mean simply importing tests validated in other regions. The continent is home to a wide range of genetic variations that influence how diseases develop. Without local validation, there is a real risk of false positives, missed diagnoses, and wasted resources. And this validation process offers an opportunity to gain deeper insights into cancer biology, which can benefit not only African populations but the global community as well.
As recent reviews conclude, the discovery and validation of novel, highly specific biomarkers for early detection remain urgent global priorities [8]. For Africa, the challenge is even more pressing. This makes investment in Africa-based biomarker research essential, not only for scientific equity but also for accuracy, cost-effectiveness, and broad clinical relevance.
Earlier this year, Metaphor documented age-specific trends for conventional biomarkers such as PSA, CA-125, CA 19-9, CA 15-3, and CEA in cancer patients in Lagos. The study’s unique value is that it provides locally generated data on when biomarker elevations occur in Nigerian populations [14]. However, because the analysis was performed in already-diagnosed patients, it cannot yet answer the question of whether these biomarkers rise early enough in asymptomatic individuals to function as true screening tools.
What it does make clear is that Africa is building capacity to generate its own cancer biomarker evidence and that such data are essential if screening strategies are to be both relevant and effective for local populations, whether home or abroad.
A parallel lesson can be drawn from expanded carrier screening (ECS), which was originally developed using predominantly European datasets. As a result, the earliest ECS panels systematically under-identified carriers of genetic conditions more frequent in African, Asian, and other underrepresented populations [15].
Just as ECS has been restructured to include more representative genetic variants, biomarker research for early cancer detection must deliberately integrate Africa’s extraordinary diversity to ensure reliability and global relevance [16, 17].
As we study oncology biomarkers in our unique population for early detection, there is potential to improve treatment as well. Metaphor’s prospective pilot study on the biological characteristics of breast cancer in Nigerian women took a step in this direction, documenting the unique prevalence of triple-negative breast cancer (TNBC) in this population, which is relevant for informing more optimal treatment pathways [18].
And McCormack et al. projected a substantial absolute survival gain of up to 12% in breast cancer patients through improvements in treatment [19]. Their findings suggest that the survival benefits of enhanced treatment are comparable to those of early detection. As a region, it is important that we prioritize the development and scaling of the technologies necessary to be part of ongoing research to enjoy this two-fold benefit of biomarker studies: for potential early detection and informing better treatment pathways.
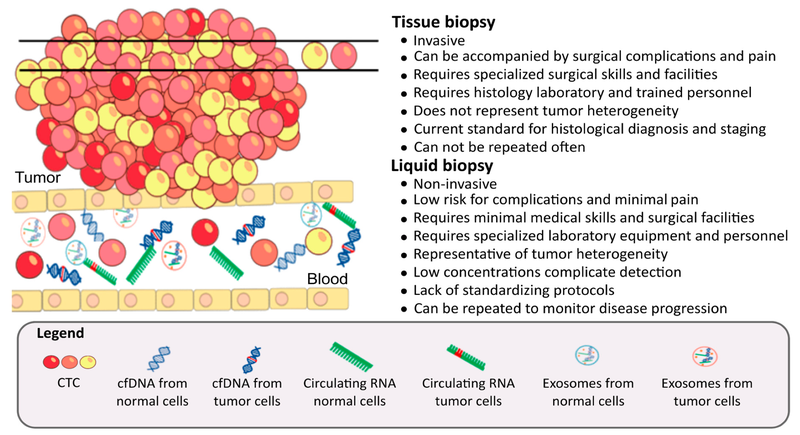
Speaking of the technologies necessary for ongoing research, in recent years, tech advancements have transformed how biomarkers are detected and used in cancer diagnostics. The future of cancer biomarkers is increasingly tied to multi-omics approaches, where data from genomics, transcriptomics, proteomics, and metabolomics are integrated. This dramatically improves the sensitivity and specificity of early detection tests [20].
One compelling example is a pilot study that used extracellular vesicle (EV) protein profiles and machine learning to detect early-stage pancreatic, ovarian, and bladder cancers with very high accuracy [21]. Artificial intelligence (AI) is also being leveraged to detect cancer risk earlier.
Liquid biopsy technologies, though still emerging, are rapidly evolving. New methods can detect exosomes from as little as 2 µL of plasma, distinguishing cancer patients from healthy controls with promising specificity [22]. It is clear from different reviews that liquid biopsy is maturing as a minimally invasive tool for early detection and disease monitoring [23, 24].
Together, these innovations are reshaping what “early detection” could mean, such that we can intercept cancer at molecular inception rather than waiting for structural changes or symptoms. It is worth supporting local stakeholders in building capacity for this tech so that advancements are inclusive.
Policy and Implementation Must Bridge the Gap
Scientific breakthroughs only save lives when they reach patients. In low- and middle-income countries, particularly across Africa, translating biomarker advances into practice requires Africa-led validation studies grounded in local genetic diversity and cancer burden. Collaborative research platforms like H3Africa demonstrate how shared regional infrastructure and capacity building can support such validation efforts [25].
Effective translation will also depend on policy frameworks that incentivize industry-academic partnerships and transparent financing models, which may include pooled procurement to reduce assay costs—essentially leveraging the economies of scale possible with our large African population.
Embedding biomarker testing into existing health programs also provides pragmatic entry points for scalability. Without intentional integration of discovery, equity, and policy, the gap between potential and impact will persist. But with coordinated investment and policy innovation, Africa can lead the shift toward proactive oncology.
As a Contract Research Organisation based in Africa, Metaphor is committed to supporting biomarker-driven research through collaborations and bold initiatives.
References
1. Ahmad, A., Imran, M., and Ahsan, H. (2023). Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics, 15(6), 1630. https://doi.org/10.3390/pharmaceutics15061630
2. Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 74(3), 229-263. https://doi.org/10.3322/caac.21834
3. Bentley, A. R., Callier, S., and Rotimi, C. N. (2017). Diversity and inclusion in genomic research: Why the uneven progress? Journal of Community Genetics, 8(4), 255. https://doi.org/10.1007/s12687-017-0316-6
4. Das, S., Dey, M. K., Devireddy, R., and Gartia, M. R. (2024). Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors, 24(1), 37. https://doi.org/10.3390/s24010037
5. Lawler, M., Keeling, P., Kholmanskikh, O., Minnaard, W., Moehlig-Zuttermeister, H., Normanno, N., Philip, R., Popp, C., Salgado, R., Santiago-Walker, A. E., Trullas, A., Van Doorn-Khosrovani, S. B. V. W., Vart, R., Vermeulen, J., Vitaloni, M., and Verweij, J. (2024). Empowering effective biomarker-driven precision oncology: A call to action. European Journal of Cancer, 209, 114225. https://doi.org/10.1016/j.ejca.2024.114225
6. Henry, N. L., and Hayes, D. F. (2012). Cancer biomarkers. Molecular Oncology, 6(2), 140–146. https://doi.org/10.1016/j.molonc.2012.01.010
7. Zhang, R., Siu, M. K., Ngan, H. Y., and Chan, K. K. (2021). Molecular Biomarkers for the Early Detection of Ovarian Cancer. International Journal of Molecular Sciences, 23(19), 12041. https://doi.org/10.3390/ijms231912041
8. Tappia, P. S., and Ramjiawan, B. (2023). Biomarkers for Early Detection of Cancer: Molecular Aspects. International Journal of Molecular Sciences, 24(6): 5272. https://doi.org/10.3390/ijms24065272
9. Uttley, L., Whiteman, B. L., Buckley Woods, H., Harnan, S., Taylor Phillips, S., and Cree, I. A. (2016). Building the evidence base of blood-based biomarkers for early detection of cancer: A rapid systematic mapping review. eBioMedicine, 10, 164–173. https://doi.org/10.1016/j.ebiom.2016.07.004
10. Cree, I.A. (2011). Improved blood tests for cancer screening: general or specific? BMC Cancer 11, 499 . https://doi.org/10.1186/1471-2407-11-499
11. George, S. H., Medina-Rivera, A., Idaghdour, Y., Lappalainen, T., and Romero, I. G. (2023). Increasing diversity of functional genetics studies to advance biological discovery and human health. The American Journal of Human Genetics, 110(12), 1996-2002.
12. Meka, Ijeoma A.; Okwor, Chika J.; Arum, Ekene J.; Ogamba, Michael I.1; Omotowo, Babatunde I.2; Kanu, Okezie O.3. Evaluation of Prostate-specific Antigen Testing: An Empirical Survey of Laboratories in Nigerian Tertiary Care Centers. International Journal of Medicine and Health Development 27(3): 233-237
13. Aldrighetti, C. M., Niemierko, A., Van Allen, E., Willers, H. and Kamran, S. C. (2021). Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies. JAMA Netw Open. 4(11):e2133205.
14. Animashaun, T., Ekpunobi, N., Idowu, O., and Obidi, N. (2025). Evaluating The Age-Specific Prevalence of Prognostic Cancer Markers Among Cancer Patients in Lagos Nigeria.
15. Johansen, T. K., Ben-Shachar, R., Torres, R., Arjunan, A., Muzzey, D., Kaseniit, K. E., Goldberg, J. and Brown, H. (2022). A guidelines-consistent carrier screening panel that supports equity across diverse populations. Genetics in Medicine, 24(1), 201–213.
16. Rotimi, S. O., Rotimi, O. A., and Salhia, B. (2021). A review of cancer genetics and genomics studies in Africa. Frontiers in Oncology, 10, 606400. https://doi.org/10.3389/fonc.2020.606400
17. Bentley, A. R., Callier, S. L., and Rotimi, C. N. (2020). Evaluating the promise of inclusion of African ancestry populations in genomics. npj Genomic Medicine, 5(1), 5. https://doi.org/10.1038/s41525-020-0116-6
18. Animashaun, T., Ekpunobi, N., Amukele, T., Ogunsanya, T., Aina, T., Idowu, O., … and Obidi, N. (2024). Evaluation of the biological characteristics of breast cancer in women from Nigeria: A non-interventional pilot study.
19. McCormack, V., McKenzie, F., Foerster, M., Zietsman, A., Galukande, M., Adisa, C., Anele, A., Parham, G., Pinder, L. F., Cubasch, H., Joffe, M., Beaney, T., Quaresma, M., Togawa, K., Abedi-Ardekani, B., Anderson, B. O., Schüz, J., and dos-Santos-Silva, I. (2020). Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. The Lancet Global Health, 8(9), e1203–e1212.
20. Milner, D. A. Jr and Lennerz, J. K. (2024). Technology and Future of Multi-Cancer Early Detection. Life (Basel). 14(7):833.
21. Hinestrosa, J.P., Kurzrock, R., Lewis, J.M. et al.(2022). Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun Med 2, 29 . https://doi.org/10.1038/s43856-022-00088-6
22. Ma, L., Guo, H., Zhao, Y. et al. (2024). Liquid biopsy in cancer: current status, challenges and future prospects. Sig Transduct Target Ther 9, 336 . https://doi.org/10.1038/s41392-024-02021-w
23. Connal, S., Cameron. J. M., Sala, A., Brennan, P. M., Palmer, D. S., Palmer, J. D., Perlow, H., Baker, M. J. (2023). Liquid biopsies: the future of cancer early detection. J Transl Med. 21(1):118.
24. Feng, Y., Yang, W., Zhu, J., Wang, S., Wu, N., Zhao, H., and Yang, X. (2025). Clinical utility of various liquid biopsy samples for the early detection of ovarian cancer: A comprehensive review. Frontiers in Oncology, 15, 1594100. https://doi.org/10.3389/fonc.2025.1594100
25. Obiora, O. L., Shead, D. A., and Olivier, B. (2024). Data sharing considerations and practice among health researchers in Africa: A scoping review. Digital Health, 10, 20552076241290955.
26. Oh, D. and Bang, Y. (2019). HER2-targeted therapies — A role beyond breast cancer. Nature Reviews Clinical Oncology, 17(1), 33-48.
27. Temilola, D. O., Wium, M., Coulidiati, T. H., Adeola, H. A., Carbone, G. M., Catapano, C. V., and Zerbini, L. F. (2019). The Prospect and Challenges to the Flow of Liquid Biopsy in Africa. Cells, 8(8), 862. https://doi.org/10.3390/cells8080862