Jan 9•Damilola Omotola, Anthony Olayemi, Tunde Animashaun
Staining is the endpoint of histological examination, and every step that came before it in the experimental process has an impact on this stage. Before being stained, histological samples are fixed, processed, embedded, and sectioned; each of these procedures has the potential to improve or worsen the stain quality (Troiano et al., 2009). A number of variables that impact the staining processes that must be closely watched include product and stain supplier modifications (hematoxylin and eosin stains are commonly used), pH consistency, stain age, and stain convention (Alghamdi et al., 2021). In the lab during the staining technique, careful attention to appropriate controls and commitment to quality control must be a top focus to achieve optimal stain quality (Vyberg and Nielsen, 2016) .
Hematoxylin and Eosin (H and E) staining, a venerable technique in the field of histopathology, stands as a cornerstone in the microscopic examination of tissues ( Bancroft and Gamble ,2008).The adaptability and broad applicability of H and E staining across varieties of tissue types is what accounts for its efficacy. This method acts as the first point of entry for diagnostic inquiries, directing more in-depth analyses and research projects as needed. (Dapson and R.W. Horobin (2013)
The unparalleled effectiveness of H and E staining lies in its ability to provide vivid contrast, allowing for the meticulous analysis of cellular details and facilitating the accurate diagnosis of a diverse array of medical conditions, which is often the gold standard in the diagnosis of many diseases.( Bancroft and Gamble ,2008: Wick, 2019). Pioneered by early scientists such as Mayer and Delafield, Its longevity is a testament to its reliability and adaptability, as it seamlessly integrates with technological advancements while retaining its core principles.(Abbas et al., 2014).
Quality control on the other hand, should be top focus as it improves the dependability of the results in the histopathological procedures by assisting in the identification and correction of potential errors or variations (Bancroft and Gamble, 2008: Mubarak, 2023). All of this adds up to ensuring the highest standards of diagnostic accuracy through the combination of continuous staff training, procedure adherence, instrument maintenance, and documentation practices (Hladik & Carson, 2009). Quality control checks in a histopathology laboratory are, precise patient identification, fixation, adequate processing, appropriate embedding techniques, microtomy, undesired artifacts, and control inspection to evaluate the accuracy of particular stains.
The old computer science adage, “Garbage In, Garbage Out,” best describes the difficulties encountered in the processes involved from reception of the sample, to slide preparation. In this case, the adage emphasizes the requirement for quality control in all processes involved because accurate and efficient diagnostic tests depend on high-quality input tissue specimens (Lerch et al., 2019)
In other to achieve an effective Hematoxylin and Eosin stained slide,there are several factors in processing, dehydration, fixation, and sectioning that can affect the quality of the stained slides during microscopic examination. Also, the tissue’s pH, staining duration, reagent selection (such as bluing or differentiation hematoxylin), number of washes, and washing reagents are all procedure-specific factors. (Wick, 2019) .
We would discuss quality control in each of the tissue processing steps below:
Tissue processing aims to transform tissues fixed in formalin into material suitable to be embedded into paraffin blocks. We now know that a review of the tissue processing procedure is necessary in order to ensure that pathologists maintain the highest standards of best practices, given the documented difficulties associated to this process. It is not debatable that improper fixation and/or processing of tissue can lead to compromised diagnostic results (Zarbo, 2022: Caril et al., 2022: Lerch, 2019). Tissue processing involves: Fixation, Dehydration, Clearing, Infitration/Impregnation.
FIXATION: Fixation is the initial step in tissue processing and involves the use of fixatives to stabilize tissues and prevent degradation. The subsequent procedures, including dehydration, clearing, infiltration, embedding, microtomy, and staining, will all be insufficient if the fixation is inadequate. It will be challenging for the pathologist or histotechnologist to provide an accurate diagnosis if the tissue has been improperly processed (Ajileye and Esan, 2022). Quality Control in fixation should be ensured to achieve a good end product on the Haematoxylin and Eosin stained slides. These are QC points to note during fixation :
Fixative Selection: For routine histology, 10% neutral buffered formalin is the most commonly used fixative for H and E stained tissues. This decision should be made in accordance with the type of tissue and the analyses that will follow (Burvana et al., 2012: Bancroft and Gamble, 2008).
Quality Control: Using established procedures, evaluate and confirm the effectiveness of selected fixatives on a regular basis. (Miller, 2009).
Fixation Duration: Tissue preservation is impacted by the length of fixation. While over-fixation can result in nonspecific background staining that can be more difficult to reverse and also cause loss of immunohistochemical antigenicity, under-fixation can cause unfixed tissues to be damaged by the dehydrating fixative effect of ethanol during processing (Suvarna et al., 2012: Torlakovic et al., 2010). Extended formalin fixation causes the fixative to stay in the tissue long after the protein cross-linking has taken place, and the tissue may become overhardened as a result of this ongoing cross-linking (Carson, 2007). Overfixed tissue is less stainable, which leads to lower-quality staining. Formalin is known to decrease the amount of cationic amino groups that are available for stain binding. For instance, since eosin binds to these amino groups in tissues, the reaction of formaldehyde with the amino groups in the tissue may prevent eosin from binding to the tissue when a tissue has been over fixed. (Carson, 2007).
– Quality Control: Standardize fixation times and keep an eye on protocol adherence for quality control.
– Continually evaluate and, if necessary, modify the fixation duration. (Gamble, Bancroft, 2008).
Tissue Thickness: Tissue thickness shouldn’t be greater than 3–4 mm (Lott et al., 2023) as this will impact fixative penetration. Longer fixation times may be necessary for thick tissues.
Quality Control: Monitor and control the consistency of tissue thickness to ensure uniform fixation across samples.
Fixative Concentration: For routine histology, 10% neutral buffered formalin (NBF, or about 4% formaldehyde) buffered to pH 7 with phosphate buffer is the most commonly used fixative. This fixative can preserve tissue and cellular morphology well and effectively prevent autolysis (Kiernan, 2000). For a fixative to be effective, its concentration must be precisely calculated. Fixatives that are too diluted may not provide enough preservation.
Quality Control: In accordance with the type of fixative being used and the tissues being processed, check and modify fixative concentrations on a regular basis., also measure the PH of the fixative used.
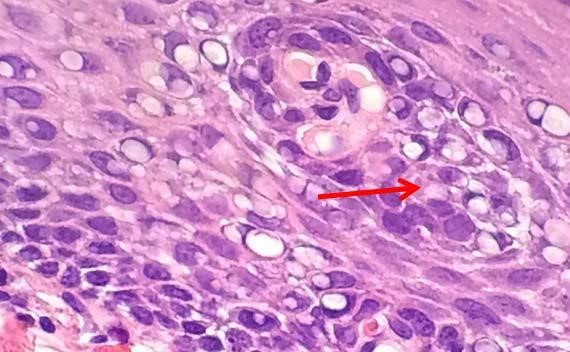
Figure 1: The bubbling is clear in this image. High heat and acidic formalin can cause protein coagulation that may impair diagnosis.
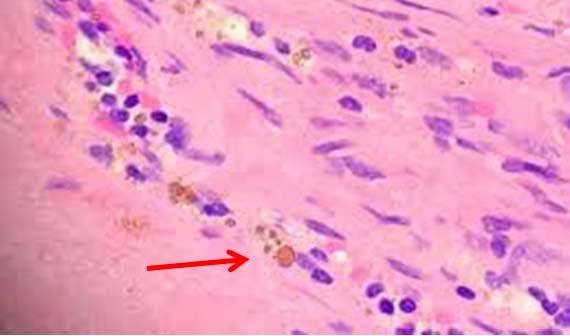
Figure 2: Hemosiderin present in this slide due to wrong fixatives used
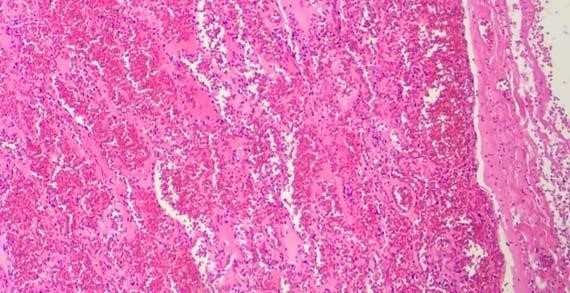
Figure 3: Cracking of the RBCs can be easily seen from lower magnification due to overly-processed tissues.
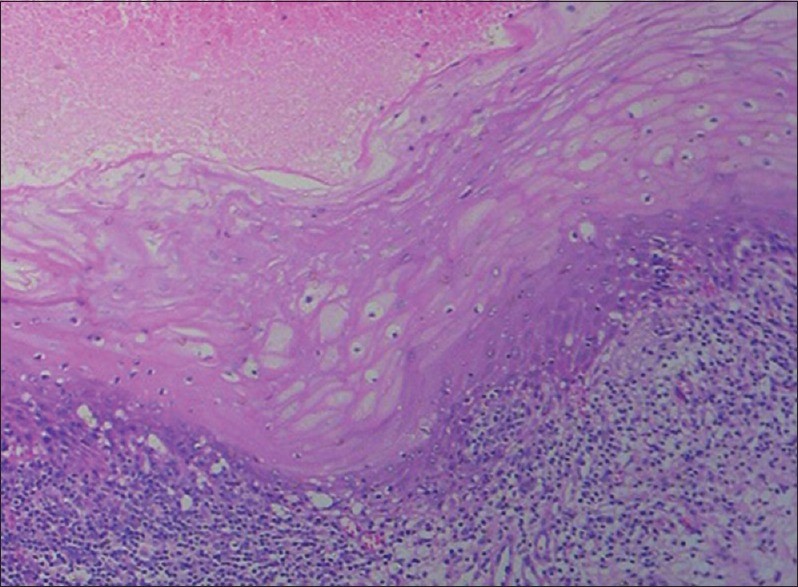
Figure 4: Histopathological image shows tissue autolysis due to delayed fixation.
DEHYDRATION: Dehydration is the process of taking the water out of biological specimens so that they can be prepared for further processing for microscopy. Insufficient dehydration leads to insufficient embedding medium infiltration and polymerization (Christian and Stadtlände,2006). Ethanol is perhaps the most widely used dehydration agent because it is less toxic, less volatile, does not extract bound lipids as much as acetone does, causes less swelling at the beginning of the dehydration process and less shrinkage at the end. Other dehydranting substances include acetone, propylene oxide, dimethoxypropane, and methanol. Swelling occurs in most tissues when dehydration is initiated, and it is offset somewhat by shrinkage as soon as the higher concentrations of the solvents are approached. In order to minimize the side effects of dehydration agents, it is recommended that the total time used for performing the entire dehydration procedure should be kept as short as possible. With extended ethanol fixation, the tissue can also be over-hardened. (Carson, 2007). It should be noted that cell shrinkage due to extended ethanol fixation or extended dehydration processing can result in cell morphology alterations (Carson, 2007).
Quality Control:
– Dehydrate tissues gradually by applying several alcohol solutions.
– To avoid hardening and artifacts, keep an eye on the duration and concentration of dehydration.
– Because dehydrants are hygroscopic, keep their bottles tightly closed to prevent water from seeping inside.
CLEARING: The tissues become transparent and clear after clearing. This phrase describes the tissues’ appearance following the removal of the dehydrating agent. The tissue turns transparent if the clearing agent’s refractive index is comparable to that of the tissue’s protein. When the tissue becomes transparent, the clearing process is finished and the alcohol is removed, completing the paraffin impregnation. According to Alwahaibi et al. (2018), clearing typically serves as a solvent for the mounting media, improving the refractive index and making the tissues transparent. This facilitates the microscopic examination of Hematoxylin and Eosin-stained slides.
Quality Control:
– Tissues should be made transparent for infiltration by clearing them with an appropriate clearing agent (such as xylene).
– Check for appropriate clearance periods and make sure enough alcohol is removed.
IMPREGNATION/INFILTRATION AND EMBEDDING: The process of impregnation involves the diffusion of a clearing agent, which allows molten wax to infiltrate the tissue and gets deposited (Bancroft and Gamble, 2008). On the other hand, embedding refers to the process of surrounding tissues with a medium, such as wax, agar, or gelatin, to provide support during sectioning (Ubalei et al., 2021).
The specimens need a long period of fixation, decalcification, dehydration, clearing, impregnation, or infiltration before they can be embedded. Because each step depends on the others, failing in one will have a direct impact on the quality and ease of sectioning, which could result in a poor stain output. Therefore, it is crucial that a technician who is responsible, cautious, and patient completes every processing step (Ubalei and colleagues, 2021). Paraffin wax denatured from excessive heating or reheating causes a breakdown of the wax molecule so that it loses its elasticity and ability to adhere to other molecules. As a result, the wax breaks down and becomes extremely crumbly. For example, using a high melting point wax in a cold climate can cause the wax to become nearly brittle and crumble due to its hardness; conversely, using a low melting point wax in a warm climate will result in the wax being extremely soft (Ellis, 2002).
Quality Control:
– Introduce a solidifying media, such as paraffin, into the tissues so they can be sectioned.
– Verify infiltration temperatures and times for best outcomes.
– For precise sectioning, position tissues in embedding molds correctly.
– Verify that the tissues are firmly embedded in the embedding medium.
MICROTOMY
Many common issues with traditional microtome sectioning can be found, such as the formation of curling sections and sections that adhere to the blade. These issues make it difficult to obtain high-quality sections, which has an impact on the Hematoxylin and Eosin staining procedure. Because microtomy affects the contrast and sharpness of the slide’s microscopic image, it must be flawless with uniform section thickness for the best possible detection of tissue abnormalities under microscope (Dong et al., 2020). Thick and thin sections, chatter, “exploding” tissues, and floaters from the water bath are all artifacts of sectioning that may negatively impact how the tissue picks up the stain.
Quality Control:
– Section tissues using a microtome to produce thin slices for mounting on slides.
– Regularly change and maintain microtome blades for precision.
– Conduct routine checks on the microtome to ensure proper functionality.
– Establish and enforce protocols for maintaining consistent section thickness
– Embedding medium must be of good quality to provide optimal support and sectioning
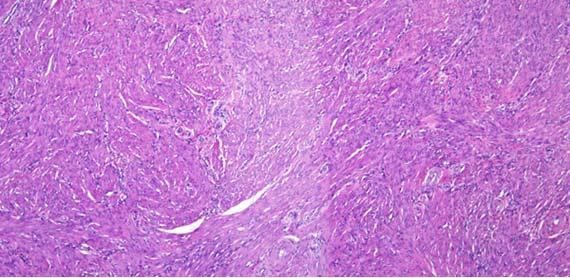
Figure 5: Note difference in coloration between these identical sections (Figure 1). Thick and thin sections can also be seen within the same tissue section.
STAINING
The actual staining process, which first involves the clearing excess wax from the tissue with xylene, hydrating in descending grades of alcohol, dipping the tissue section in hematoxylin which stains nucleic acids in a blue-purple color,differentiating with acid alcohol and bluing . Afterwards, the tissue is then counter-stained with eosin which marks proteins non-specifically within the cytoplasm, borders of the cell membrane, red blood cells and extracellular structures (including collagen) in varying degrees of pink and finally dehydrated with ascending grades of alcohol(Radu et al., 2021). Aside from the variables that can influence the success of a well-executed hematoxylin and eosin staining technique on tissue, this article also discusses variables that can influence the staining procedure’s result. Several factors that affects the staining procedures which is essential to be monitored are; modification of product and supplier of the stains (hematoxylin and eosin stains used in routine), consistency in pH, the oldness of the stain in addition to the degree of its convention, the tissue’s pH, staining duration, reagent selection (such as bluing or differentiation hematoxylin), number of washes, and washing reagents (Alghamdi et al., 2021: Wick, 2019).
Modification of Product and Supplier of the Stains: The staining result can be affected by altering the staining reagent formulation or using a different supplier ,and to ensure consistency in results, standard protocols must be established with particular stain formulations and suppliers.
Consistency in pH: When staining, pH is very important, especially when using dyes like hematoxylin and eosin. Changes in pH can have an impact on a dye’s affinity for cellular structures. Reproducibility requires routinely checking and adjusting the pH levels in staining solutions.
Tissue’s pH: The uptake and binding of dyes can be affected by the pH of the tissues being stained. Achieving consistent results requires understanding the properties of the tissue being stained and ensuring proper fixation, as tissue fixation techniques can alter pH.
Number of Washes: Standardizing the number of washes will help prevent variations in background clarity and staining intensity and this would ensure eliminate surplus stain and avoid background artifacts.
Choice of Reagents: Selecting buffer solutions or distilled water as washing reagents can affect how much excess stain is removed. Staining tissues requires the use of reliable, high-quality washing reagents to preserve their integrity.
Quality Control:
– Perform staining procedures according to established protocols.
– Monitor staining times and concentrations to achieve consistent results
– Filter out stains to reduce artifacts
– Change old reagents
– Always use a known control alongside staining
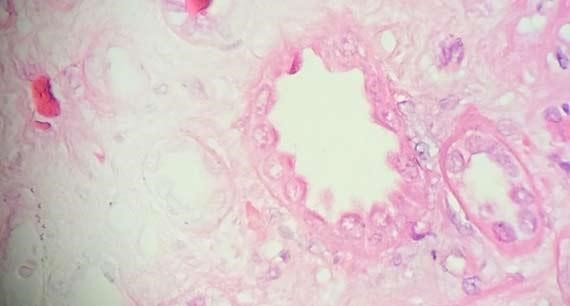
Figure 6 : The uneven staining in this section is a direct result of not enough time in in xylene to remove all of the paraffin from the tissue. This artifact is also seen when the support media for frozen sections has not been adequately remove.
When staining with hematoxylin and eosin, additional quality control procedures that ought to be followed are:
Cover-slipping:
– Apply a coverslip with the proper mounting medium;
– Verify that tissue structures are clearly visible by looking for air bubbles.
Microscopic Inspection/Slide Screening:
– Make sure that microscopes are regularly calibrated and maintained to enable precise observation.
– Examine sections for homogeneity, thickness, and the lack of creases or other irregularities.
= Adhere to established procedures when setting sectioning parameters.
– Check to make sure the sections with stains are the desired quality.
Slides for Quality Control:
– To monitor the staining quality, include known control tissues with every staining batch.
– Examine control slides on a regular basis to spot any variations in staining patterns.
Training and Competency Assessment:
– Provide ongoing training for laboratory personnel involved in histopathological procedures.
– Regularly assess and document the competency of staff members.
Documentation:
– Document each step of the tissue processing, including dates, reagent concentrations, and any deviations.
– Maintain records for traceability and quality assurance.
TROUBLESHOOTING PROCESSES
Several challenges may arise during section and staining processing as a result of potential errors in earlier procedures. A competent medical technologist should be vigilant in identifying these errors, as they could lead to complete processing failure, subpar sectioning, and ultimately inaccurate tissue evaluation if left unchecked. The most frequent issues encountered when processing tissue and during staining are listed below, along with an explanation and potential fix for each of them:
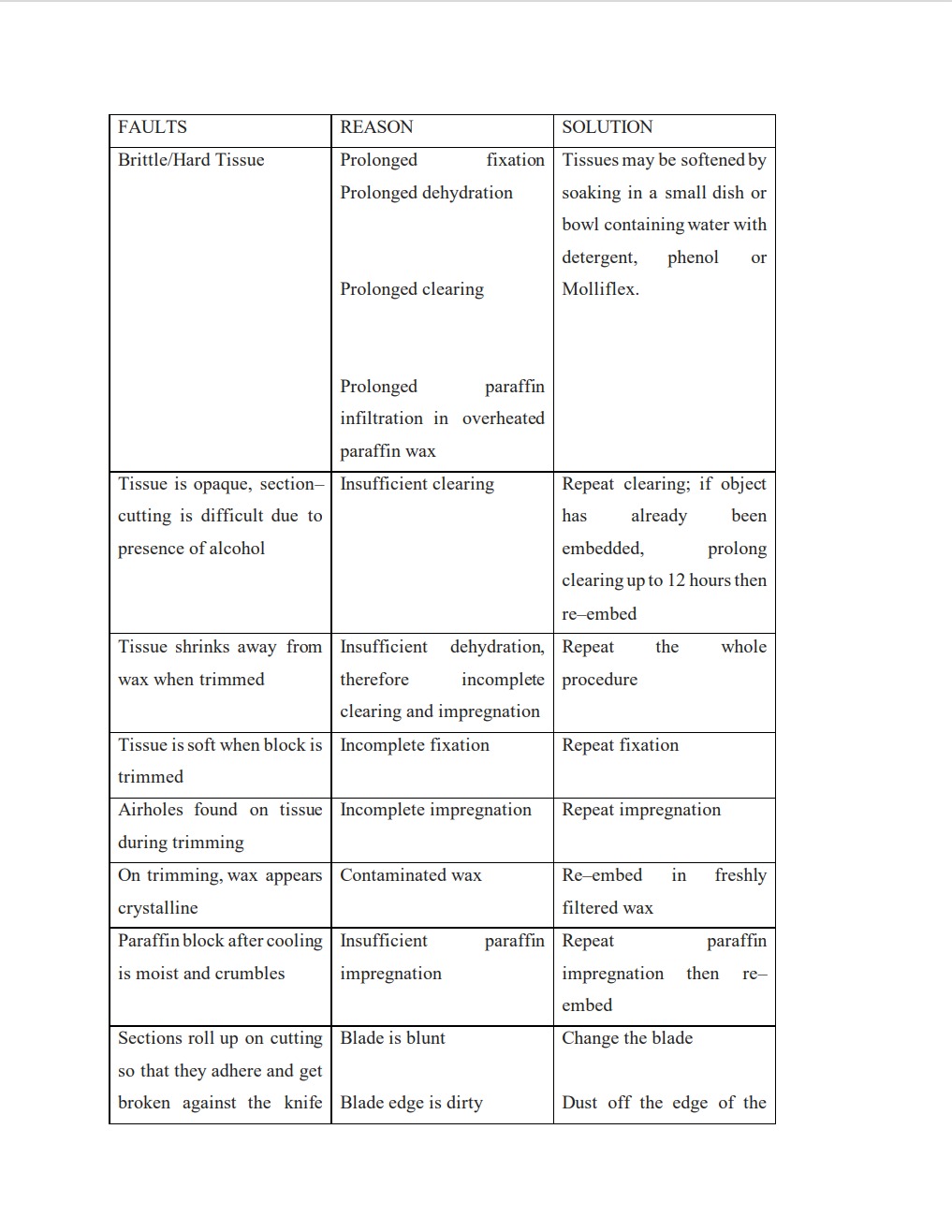
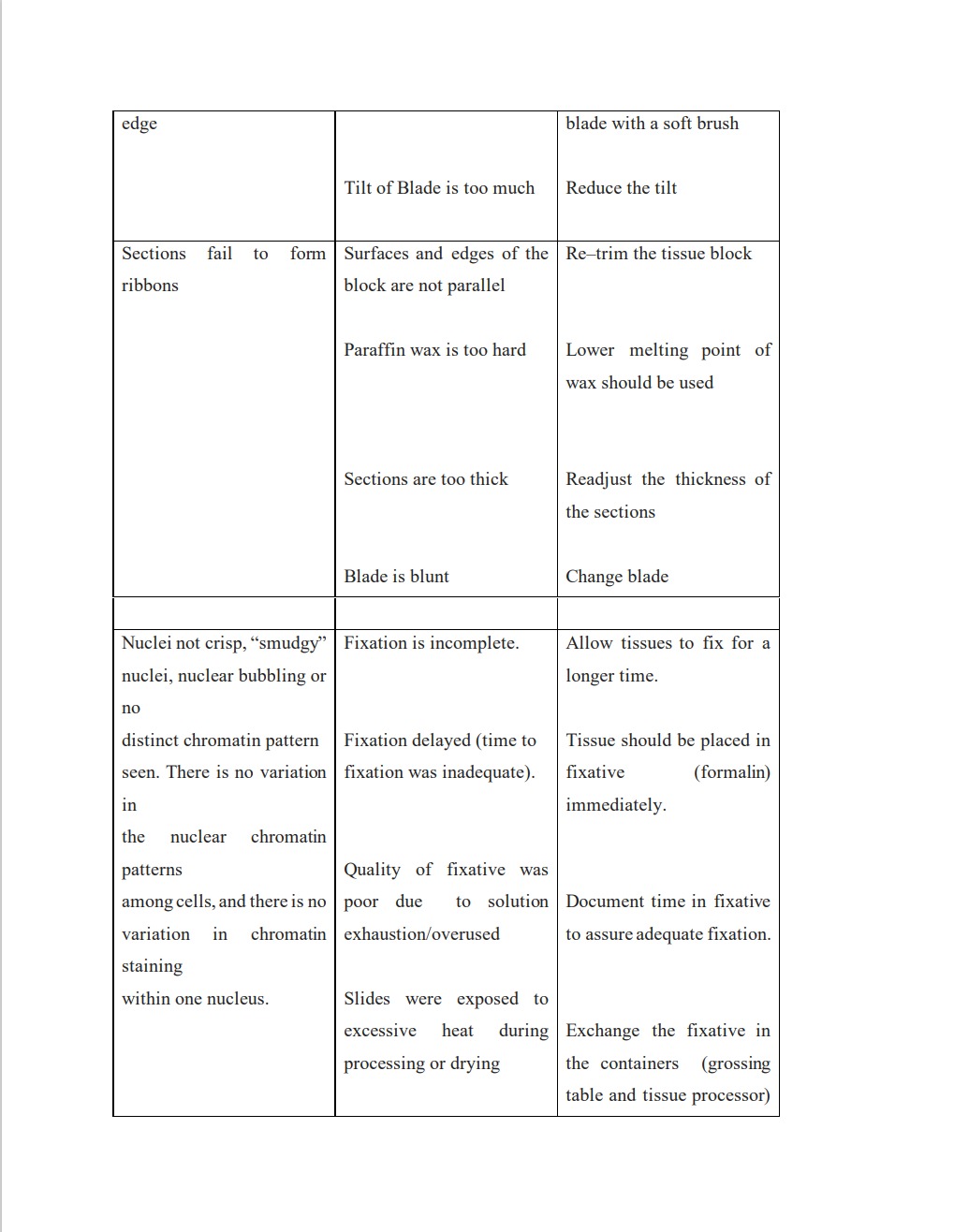
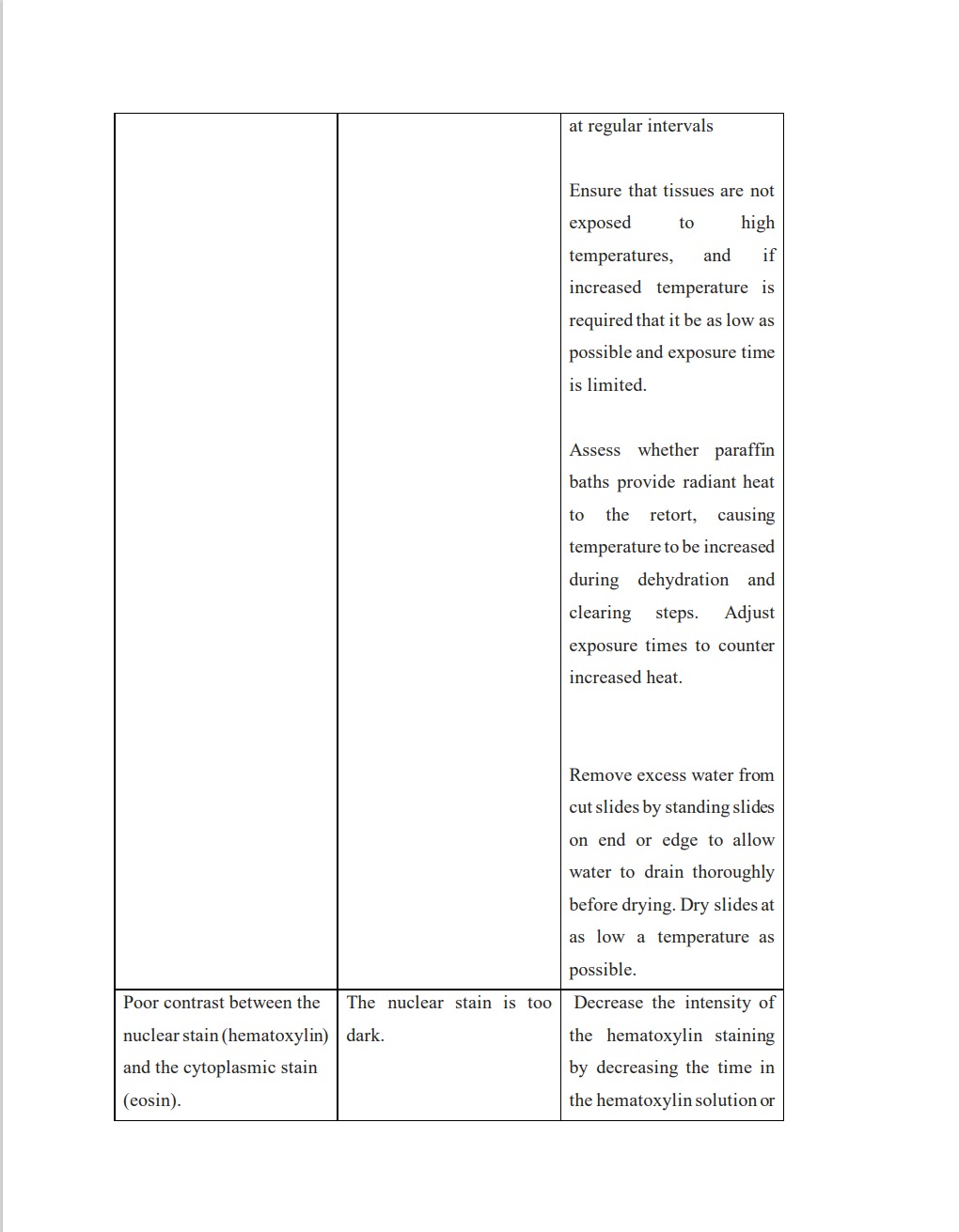
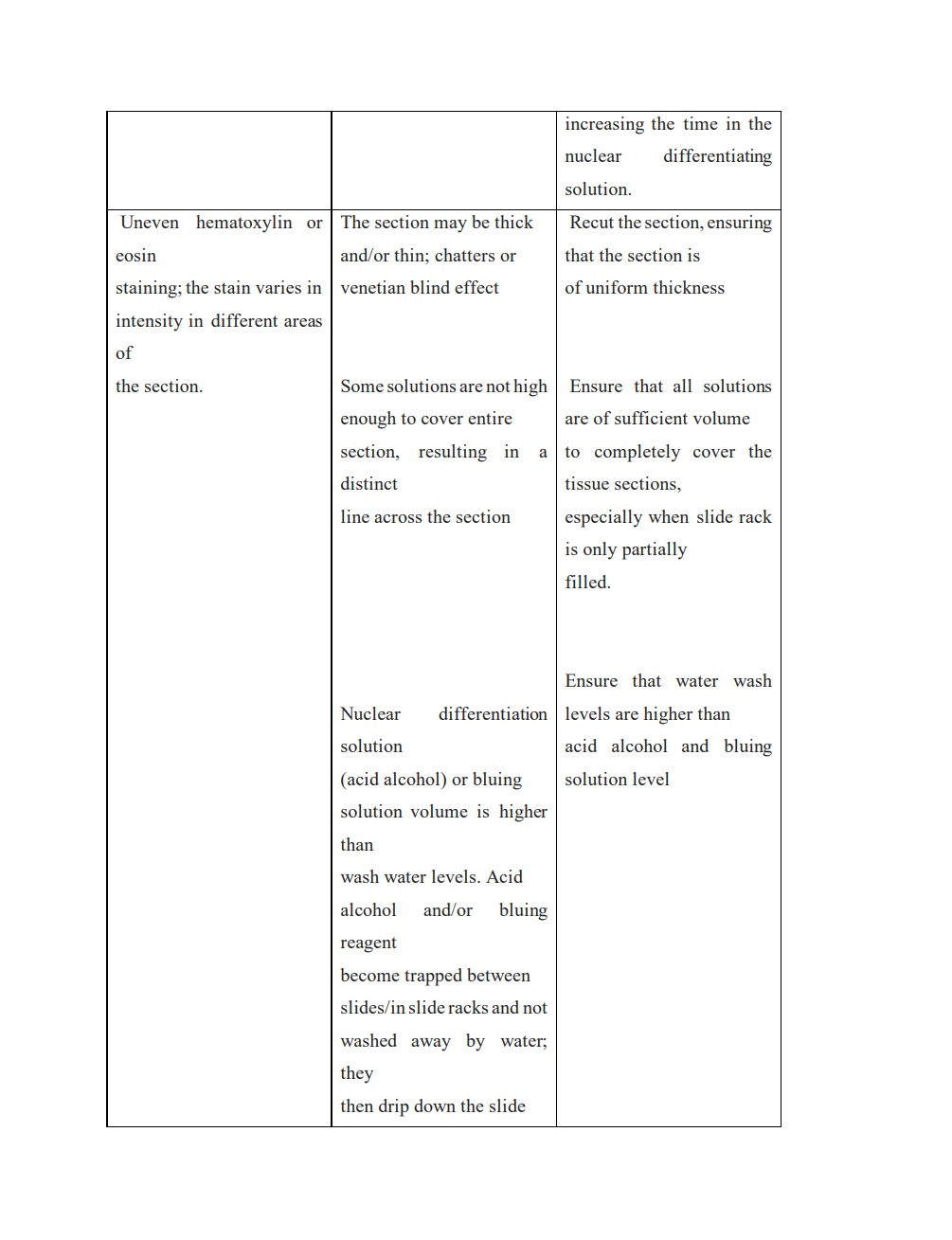
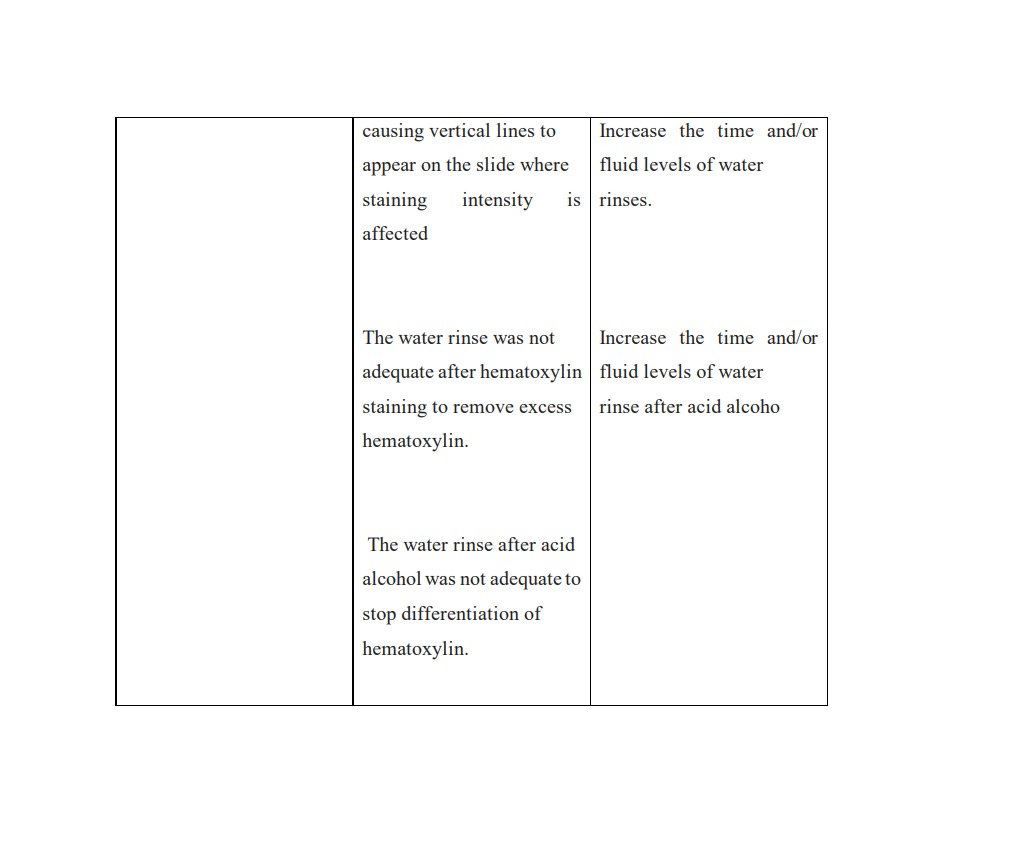
https://documents.cap.org/documents/h-and-e-troubleshooting-guide.pdf
https://mt-lectures.blogspot.com/2018/07/faults-in-processing.html
CONCLUSION
It is impossible to overestimate the importance of H&E staining in the field of pathology. This age-old method, which uses the dyes hematoxylin and eosin, has proven instrumental in providing detailed insights into the microscopic structure of tissues. Furthermore, the careful consideration of potential difficulties and variances of Haematoxyllin and Eosin stains on tissue emphasizes, the importance of meticulous laboratory practices, including ensuring optimal quality.
REFERENCES
Adyanthaya, S., and Jose, M (2013). Quality and safety aspects in histopathology laboratory.17(3): 402–407
Ajileye, A. B. and Esan, E. O (2022). Fixation and Fixatives in Histopathology: A Review.Bayero Journal of Pure and Applied Sciences, 15(1): 231 – 243
Alghamdi, R. S., Alharbi, T. S., Alsubaie, W.R ( 2021). Quality Standards of Histopathology Laboratory and Work Facilities in a Developed Country. Arch Pharma Pract;12(1):90-97
Alwahaibi, N., Aljaradi, S., and Alazri, H. (2018). Alternative to xylene as a clearing agent in histopathology. J Lab Physicians. 10(2): 189–193.
Bancroft, J.D and Gamble, M . Theory and practice of histological techniques. Churchill Livingstone Elsevier, Philadelphia, PA, 2008
Carll, T., Fuja, C., Antic, T., Lastra, R., Pytel., P(2022): Tissue contamination during transportation of formalin-fixed, paraffin-embedded blocks. Am. J. Clin. Pathol., 158, pp. 96-104
Carson F.L (2007). Histotechnology, A Self-Instructional Text. 2. Singapore: American Society for Clinical Pathology Press; . Fixation; pp. 1–24
Dong, W., Anish, R., and Vadim, S. V. (2020): Production of high-quality extremely-thin histological sections by ultrasonically assisted cutting. Journal of Materials Processing Technology, 276: 116403
Ellis, R. (2002): Porbelems in Histopathological Technique.IHC World: Life Science Products and Services.
https://documents.cap.org/documents/h-and-e-troubleshooting-guide.pdf
https://mt-lectures.blogspot.com/2018/07/faults-in-processing.html
https://www.leicabiosystems.com/knowledge-pathway/he-basics-part-4-troubleshooting-he/
Kiernan, J. A. (2000). Formaldehyde, formalin, paraformaldehyde and glutaraldehyde: What they are and what they do. Microscopy Today. 1(1). 08-12.
Leong, A.S., and Gilham, P.N. The effects of progressive formaldehyde fixation on the preservation of tissue antigens. Pathology. 1989;21(4):266-268.
Lerch, M.L. Bauer, D.R., Theiss, A., Chafin, D.,Otter, M., and Bair, G.S (2019). Monitoring Dehydration and Clearing in Tissue Processing for High-Quality Clinical Pathology. Biopreserv Biobank,17(4): 303–311.
Lott,R Tunnicliffe, J., Sheppard, E., Santiago, J., Hladik, C., Nasim,M., Zeitner,K., Haas, T.,Kohl, S., Movahedi-Lankarani, S(2023).Practical Guide to Specimen Handling in Surgical Pathology.
Mubarak, M. (2023); Quality Assurance in Histopathology Laboratories. J Clin Transl Pathol.. doi: 10.14218/JCTP.2023.00035.
Odega, K(2015): Quality Control and Assurance in Histopathology Laboratory.
Radu, H., Stefan, G.S., Adrian,D., Bogdan, P., Iustin, F., Mariana, C.,and George A. S (2021): Influence of hematoxylin and eosin staining on the quantitative analysis of second harmonic generation imaging of fixed tissue sections. 12(9): 5829–5843.
Rolls, G (2023). Steps to Better Grossing.Leica Biosystems.
Stadtländer,T. K.-H (2006). Dehydration and Rehydration Issues in Biological Tissue Processing for Electron Microscopy.
Suvarna, K.S, Layton, C. and Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques. Churchill Livingstone, 2012.
Torlakovic, E.E., Riddell, R., Banerjee, D., El-Zimaity, H., Pilavdzic, D., Dawe, P, Magliocco, A., Barnes, P., Berendt, R., Cook, D., Gilks, B., Williams, G., Perez-Ordonez, B.,
Wehrli, B., Swanson, P.E., Otis, C.N., Nielsen, S., Vyberg, M., Butany. J. (2010): Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee /Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. Am J Clin Pathol.133(3):354-365
Troiano,N.W., Ciovacco, W.A., and Kacena M.A. (2009). The Effects of Fixation and Dehydration on the Histological Quality of Undecalcified Murine Bone Specimens Embedded in Methylmethacrylate. J Histotechnol 32(1): 27–31
Ubalei, M., Gadodia, P.,Murgod, V., Patil N, Kumar, V.(2021). Journal of Interdisciplinary Dental Sciences, 10(2) 08-12
Vyberg, M. and Nielsen, S. (2016). Proficiency testing in immunohistochemistry—experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 468, 19–29.
Wick, M.R. (2019): The hematoxylin and eosin stain in anatomic pathology—An often-neglected focus of quality assurance in the laboratory. Semin Diagn Pathol 36(5):303-311.
Zarbo, R.J.(2022): The unsafe archaic processes of tissue pathology. Am. J. Clin. Pathol., 158, pp. 4-7